Introduction & strategy
Many therapeutics are nowadays produced in living cells. When biopharmaceutical companies develop new Biologics, they usually go through different steps including Bioprocess optimization, with the ultimate goal to increase yields and purity of the drug product. To obtain a better drug substance (DS), it usually requires to modify production and/or purification processes. But changing these settings could cause the opposite effect and alter product purity or efficiency. Consequently, quality control analysis and biological activity assessment are required, particularly batch comparison for Critical Quality Attributes (CQA), including Host Cell Protein analysis (HCP analysis).
In this regard, we deployed a MS-based assay called HCP-PROFILER (figure 1) to individually identify and quantify each HCP in a sample during a single LC-MS analysis. This solution is based on an innovative standard relying on a hydro-soluble bead containing an internal calibration curve including several peptides at well-defined concentration levels. Integrated software has also been developed to improve robustness of data analysis and data management. The standard is added into the sample before MS analysis, allowing to overpass instrumental variations and to do batches comparison, even if their production is several months apart. To the extent of our knowledge, this method brings a unique analytical solution compared to classical approaches, such as Enzyme-linked immunosorbent assay (ELISA), which suffer from several limits. Indeed, even if ELISA is commonly used to monitor global HCPs levels, it does not allow comprehensive HCP identification and only provides a total HCP amount in arbitrary units.

Figure 1 : HCP profiler integrated workflow
In this study, we investigated why a Biologic excreted in the extracellular media lost its catalytic activity in 1 out of 3 production batches following process scale up and why catalytic activity is present for the 4th production batch after some optimization of the large-scale upstream process.
Results
Production at R&D level compared to industrial production
In this case, to increase the drug product manufacturing yield, upstream and downstream processes were updated. First changes in upstream process (increase of both inducer quantity and fermentation time) make upstream and downstream process difficult and lead to an inactive drug product. After an identification step, according to a database search strategy, HCPs were individually identified in the first and the second small scale production batches and in the first industrial batch. HCPs analysis of this industrial batch (Batch N°3), and comparison with HCPs coming from small scales batches (Batch N°1&2) allowed the identification of a small number of new remaining HCPs involved by industrialization process implementation (Figure2). After more investigations, it was especially observed that a large part of these proteins didn’t own signal peptide necessary for protein excretion in extracellular media. Thus, by comparing these results to those obtained in batches 1 & 2, it has been demonstrated that cell lysis consequently to cell death happened during the 3rd production obviously induced by an increased incubation time during the related process.
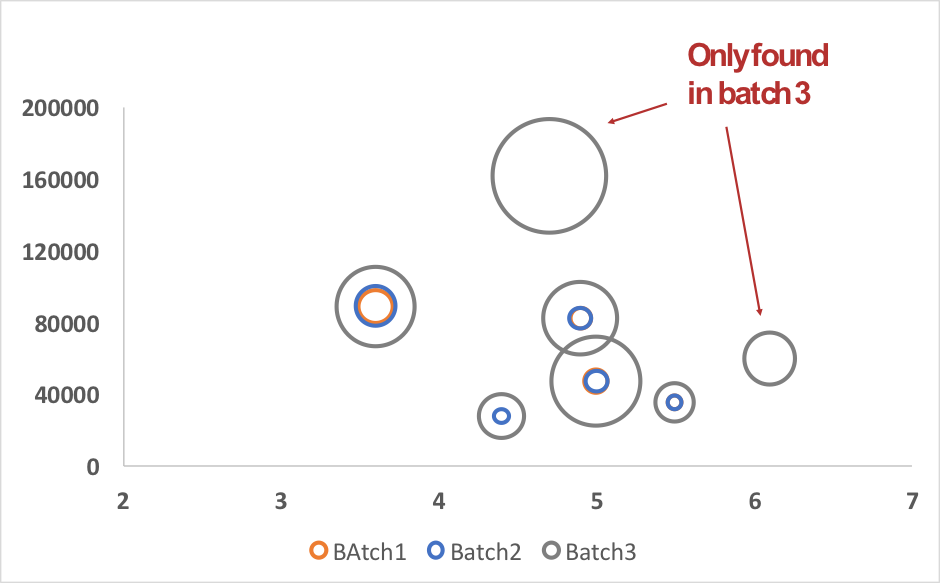
Figure 2: 2D-plot obtained for 7 proteins over-expressed in batch 3. In this plot, each circle represents individually identified proteins depending on its MW and Isoelectric point and its size corresponds to the amount of the protein.
Among these 7 differentially expressed proteins, two particularly risky HCPs owning hydrolase inhibitor activity or proteolytic activity such as the Cathepsin D were found, explaining the loss of activity of the drug product.
Improvement of industrial process to avoid an increase of HCP quantity
Secondly, an updated industrial process was implemented, the batch 4 (with increased fermentation time but with less inducer quantity that was used in small scale processes). Large scale production performances were reached whereas downstream process remained difficult. For this production, drug product activity was measured and passed QC controls. This time, even if DSP was going through with some troubles, the USP allowed the production of an active therapeutic protein. Individual HCPs were identified and quantified with HCP-PROFILER in this drug product and compared with HCPs quantified in previous batches.
Also, consequently to the difficulties encountered during the DSP, many HCPs were identified in this second industrial batch (more than 300 whereas 250 in first industrial batch or around 100 in small scale drug product batches).
HCPs identified in this Batch N°4 new industrial batch were compared with HCPs identified in the Batch N°3 in which no drug activity was detected and with the previous validated small-scale product batches (1 and 2).
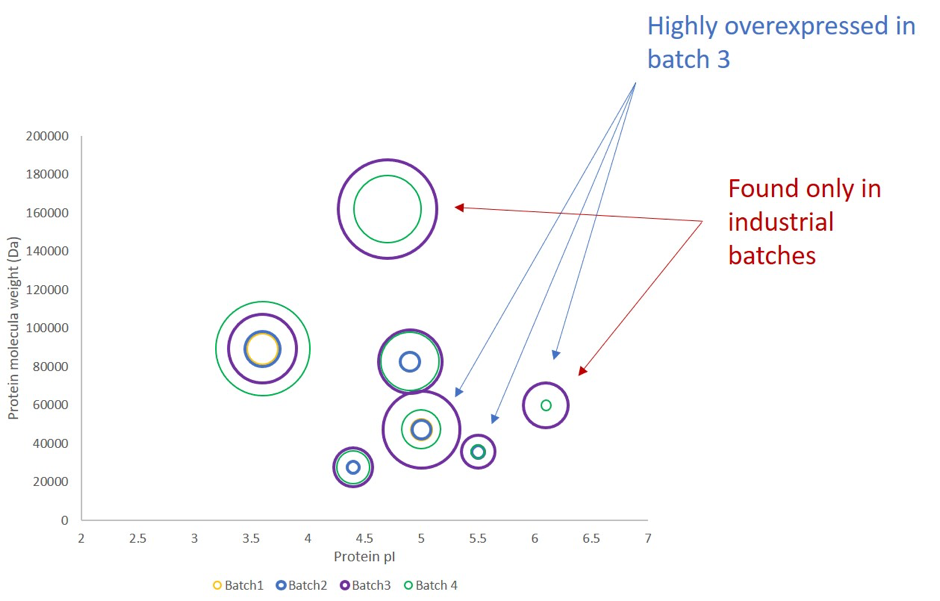
Figure 2: HCPs amount comparison between 4 batches
Regarding the new industrial batch (Batch N°4), hydrolase amount, previously differentially expressed, back up to normal amounts whereas, cathepsin D amount was still found overexpressed.
Consequently, it was confirmed that the hydrolase was involved in the loss of drug activity, after the first industrialization process whereas the cathepsin D was not. MS-based approach appeared to be an interesting way to investigate the observed lack of activity.
Concluding remark
The objective of this study was to investigate why after optimising a process, a biologic didn’t show any catalytic activity. Critical quality attributes monitoring, particularly HCPs, appeared a judicious way to explore, to assess whether risky HCPs could be incriminated. Since classical approaches such as ELISA does not allow to individually identify them, we deployed our HCP-PROFILER integrated workflow. Results demonstrated the power of this method to individually identify and quantitate HCPs in a single LC-MS-based analysis, with an easy and straightforward pipeline. Moreover, this approach provided valuable information for understanding the observed differences. First, identified proteins led to the understanding that cell lysis probably occurred during the Upstream Process (USP). Secondly, quantitative results showed that one protein was significantly over-expressed in batches 3, in which the drug lost its catalytic activity. Thirdly, batch comparison over months is ensured, thanks to the universal internal standards properties brought by HCP-PROFILER (based on READYBEADS technology). To conclude, these crucial pieces of information indicated the way to follow to push forward process development optimization and to reach the regulatory expectations.
 Anaquant HCP analysis I Protein characterisation I Protein analysis
Anaquant HCP analysis I Protein characterisation I Protein analysis