A biosimilar is a biologic product with similar biological, chemical and physical properties as the original product but that has been produced in different conditions. When the original product’s patent expires, biosimilars can be manufactured after regulatory approval. In order to prove the consistency between the biosimilar and the original product, Host Cell Proteins (that are protein impurities) must be monitored. Indeed, the biosimilar HCP profile must be shown to be as close as possible to the parent innovator biologic product. From these similarities depend the product safety.
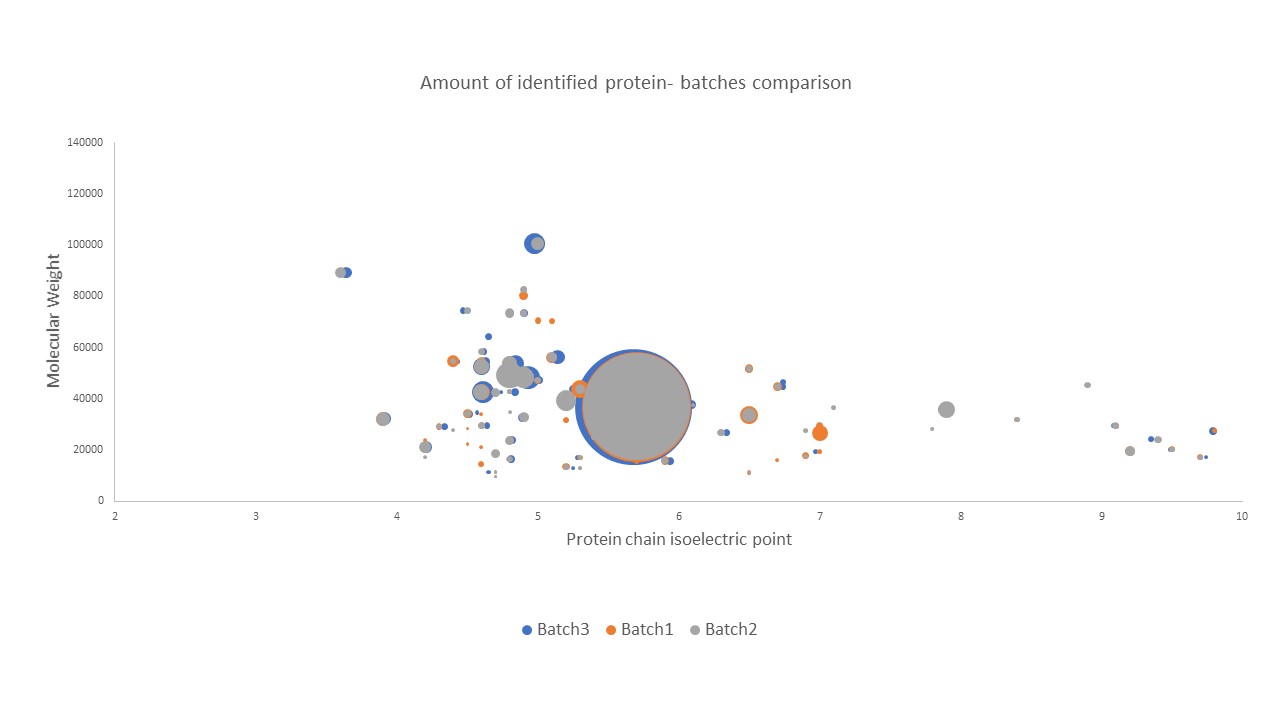
In this example, we compared different batches of biosimilar production in order to monitor the bioproduction. Each HCP was screened for identification and quantification with our solution HCP Profiler.
Our 2D plot visualization (below) allows to compare the HCPs profiles. Each spot correspond to a protein and the size circle gives the individual quantification evaluation. With this study, our customers get a detailed list of the HCPs that ensure a good monitoring of the process. The quality control included in the HCP Profiler solution allows monitoring batches through time and ensure a high consistency. Furthermore, software brings more consistency and allows a clear data visualization.
In conclusion, our HCP Profiler solution allow to:
- Compare your batches with high reproducibility and consistency
- Gain confidence in your batches
- Support your reglementary dossier
 Anaquant HCP analysis I Protein characterisation I Protein analysis
Anaquant HCP analysis I Protein characterisation I Protein analysis